Structure Of The Atom Worksheet. An factor 12X24 loses two electrons to type a cation which combines with the anion of component 17Y35 fashioned by gaining an electron. With fewer electrons than protons, the atom will have a constructive cost. Drawing Bohr Models 1. Download syllabus for Class 9 Science issued by CBSE and NCERT for 2021.
Download syllabus for Class 9 Science issued by CBSE and NCERT for 2021. Download newest curriculum with essential subjects, chapter weightage, subject wise marks… Download NCERT books for Class 9 Science, full e-book or each chapter in Science e-book for Class 9 in pdf.

Water is made up of two hydrogen atoms and one oxygen atom. These three atoms share electrons. Atoms can have just one proton or they will have multiple.
The Essential Subatomic Particles Are
How do you need to examine today? Focus your finding out with a path.

Name the three subatomic particles of an atom. Atoms can’t be created, destroyed or reworked into atoms of different components. Every factor is composed of extraordinarily small particles called atoms.
Structure Of Atoms
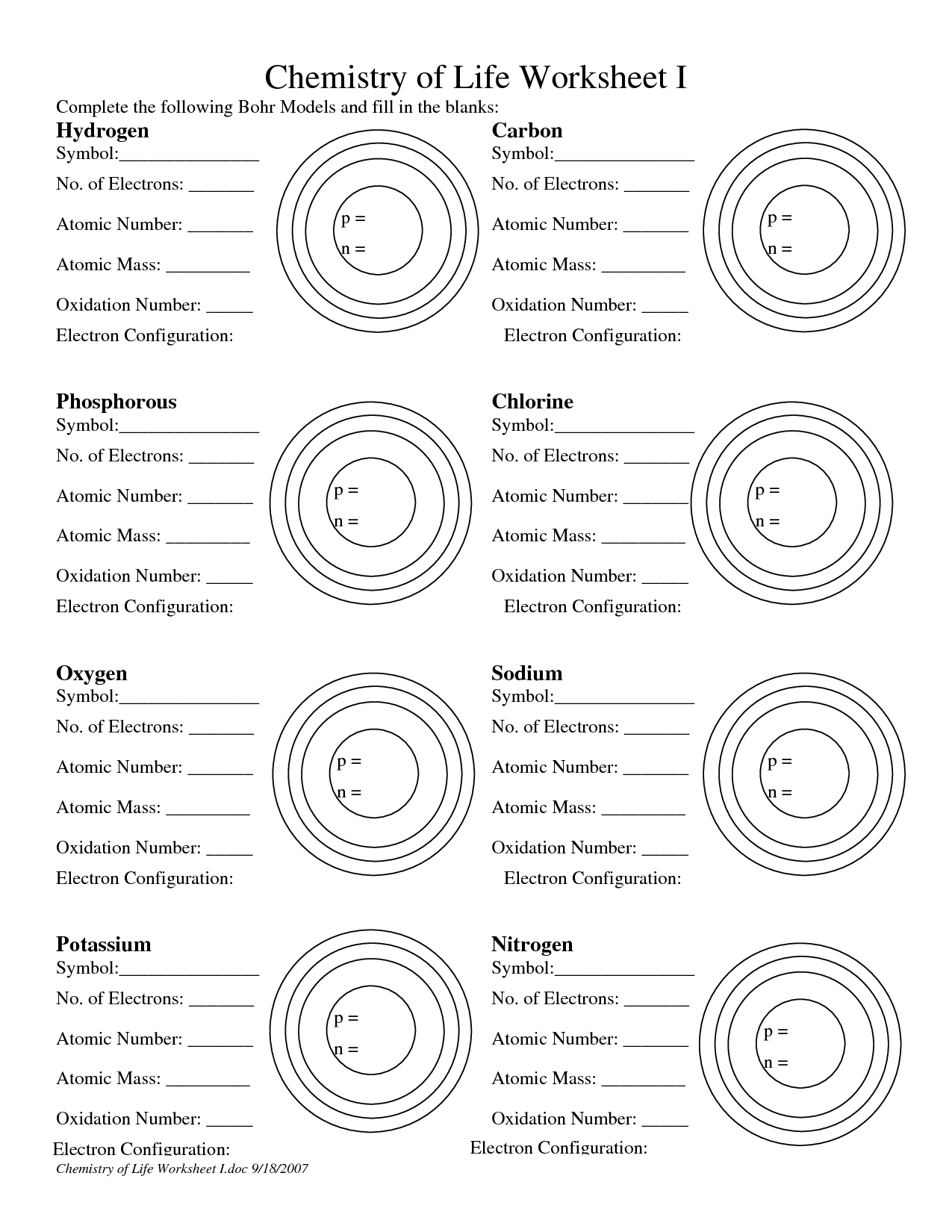
Bohr Model Practice For every component, write the total variety of electrons on the line. Then colour the right number of electrons for each orbit. Remember, fill the orbit closest to the nucleus first, however by no means exceed the quantity each orbit can maintain.
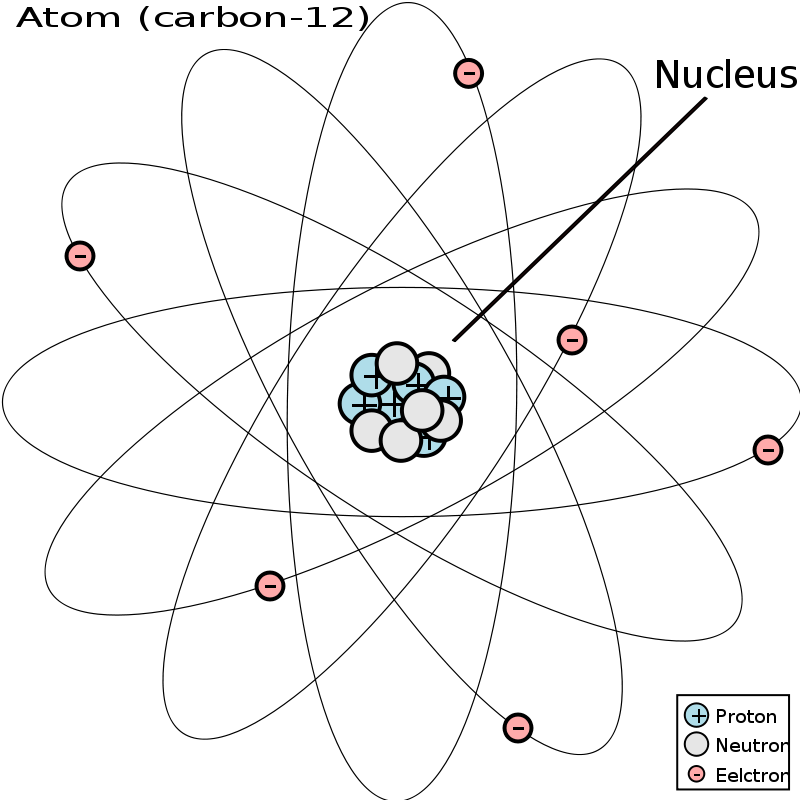
The nucleus, comprising the protons and neutrons, contributes to nearly all the mass of the atom. (Mass is the quantity of matter within the atom.) This means that electrons have actually very little mass.
Schedule A Free Session To Clear Worksheet Doubts
Iii)Whole mass of an atom is concentrated in its centre. Displaying all worksheets related to – Structure For The Atom.
At the very center of an atom is the nucleus, which is made up of small particles referred to as protons and neutrons. Protons are very small, positively-charged particles, and neutrons are impartial particles that have no cost. Some isotopes type naturally by way of changes in the nucleus of the atom.
Board Exams Date Sheet Class 10 And Class 12
Brainscape Find Flashcards Why It Works Educators Teachers & professors Content … Atomic Structures, Solubility, Priceples of chemistry 1b Show Class chemistry igcse.
Most of the alpha-particles handed straight by way of the gold foil. All isotopes of a component give equivalent chemical reactions. What are valence electrons?
A group of atoms that every one have the very same number of protons known as an element. For instance, hydrogen is a component with one proton in the nucleus and carbon is a component with 6 protons.
Cambridge IGCSE May 2018 Paper three Theory Q1 d … Atom Structure Past Paper Questions 6 Atomic structure P ast Paper Q uestions Science Exams Sorted ….
Structure For The Atom
Elaborate the postulates put forward by E. Rutherford in regards to the construction of atom primarily based on the a -particle scattering experiment. If K and L shells of an atom are full, then what can be the entire variety of electrons in the atom?
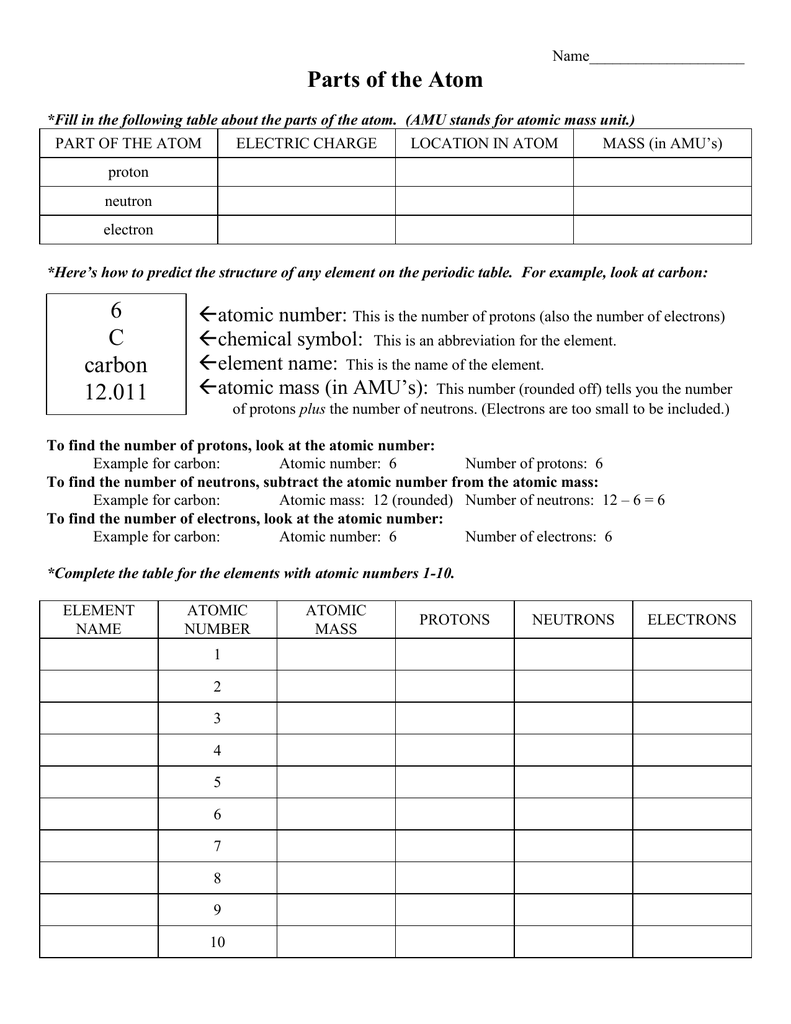
Draw the primary vitality degree. Draw the electrons within the energy levels based on the rules below. Make certain you draw the electrons in pairs.
Atoms of a given component are equivalent, each in mass and properties. Different chemical parts have totally different sorts of atoms; specifically, their atoms have completely different plenty.
Name an element which has one electron, one proton and no neutron. Isotopes of an element have identical digital configuration.
Electrons in the Bohr model comply with the variety of elements in the rows of the periodic desk. 2 in the first orbit 8 in the second and third ring 18 in the fourth and so forth… The Bohr model is a simplistic technique of explains the position of the subatomic particles of the elements of the periodic desk.
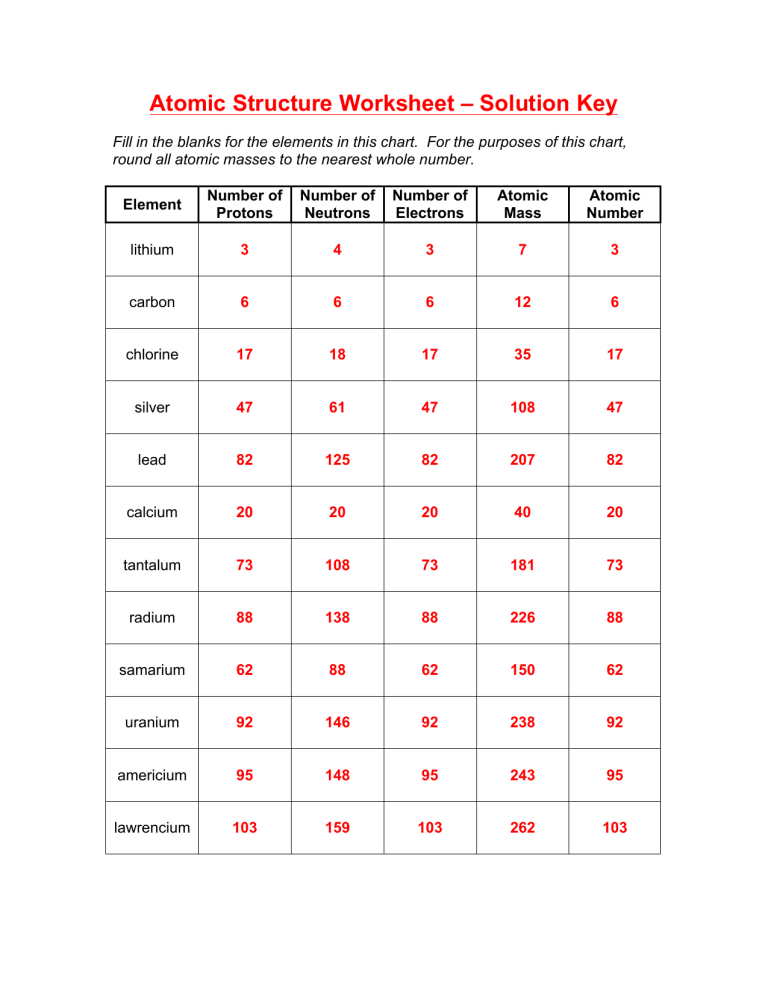
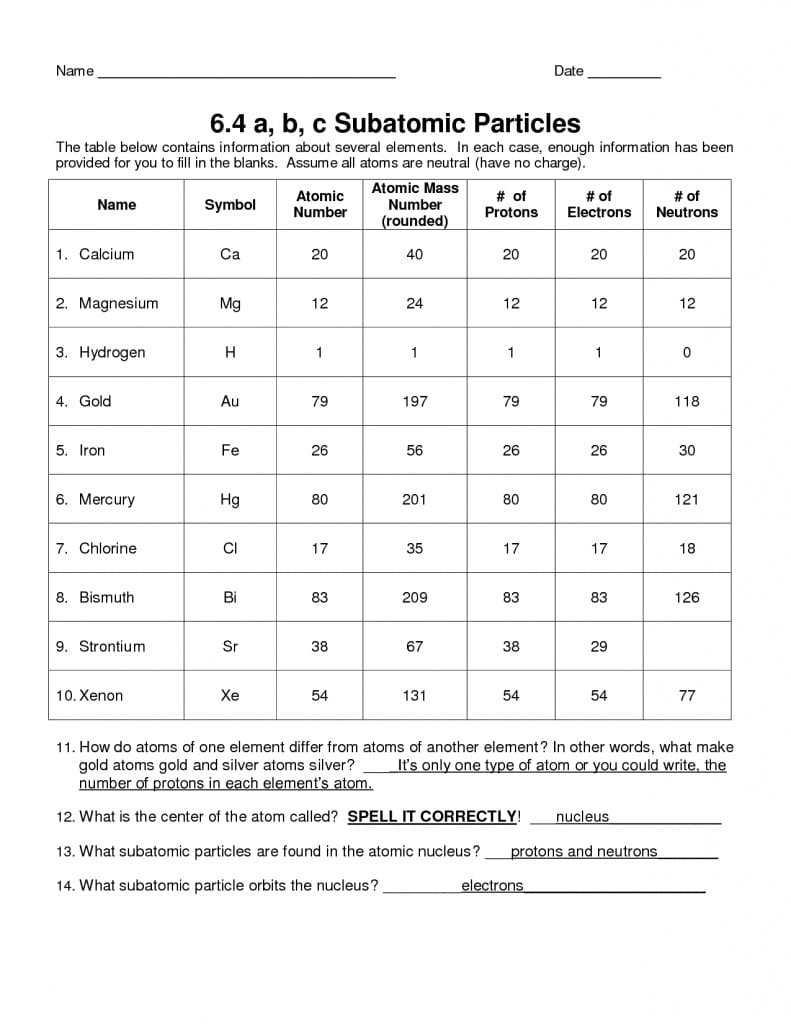
The variety of electrons will determine how atoms interact with one another and determine if the atom as an entire is constructive, adverse, or impartial. The mass variety of an atom is discovered by adding together the number of protons and neutrons. And finally, the charge of an atom is determined by the variety of protons and electrons within the atom.
The species A and B are isotopes, as they’ve identical atomic quantity but totally different mass quantity. For answers/solutions to any question or to be taught ideas, take aFREE TRIAL Session.

Free Sample Papers with solutions for Class 9 Science, obtain… An component 12X24 loses two electrons to form a cation which combines with the anion of element 17Y35 shaped by gaining an electron. Almost the entire mass of the atom is concentrated within the nucleus.

There are many properties of atoms to know and study. The tiniest bit of anything is the atom. The tiniest bit of gold is one atom of gold.

The paths of these electrons are fully random. Even the protons and neutrons continuously move at random contained in the nucleus.

Take a practice take a look at. Get quicker at matching phrases.

What is the valency of this element? Describe the essential properties of the atomic nucleus. Compare these properties with the properties of electron.
The cost of an atom is calculated based on the distinction between the number of protons in the nucleus and the variety of electrons orbiting the nucleus. Atoms have a sure variety of electrons orbiting the nucleus.
- It indicates that the optimistic charge of the atom occupies a very little house.
- Take a practice test.
- Download HOTs Questions for Class 9 Science for all important matters in Class 9 Science based on CBSE NCERT syllabus and latest sample.
- Our expert science tutors break down the matters via interactive one-to-one sessions.
- The study of isotopes is of immense importance to areas corresponding to Biology, Medicine, Earth & Planetary Sciences, and a lot of extra.
- The species A and B are isotopes, as they have identical atomic quantity however totally different mass number.
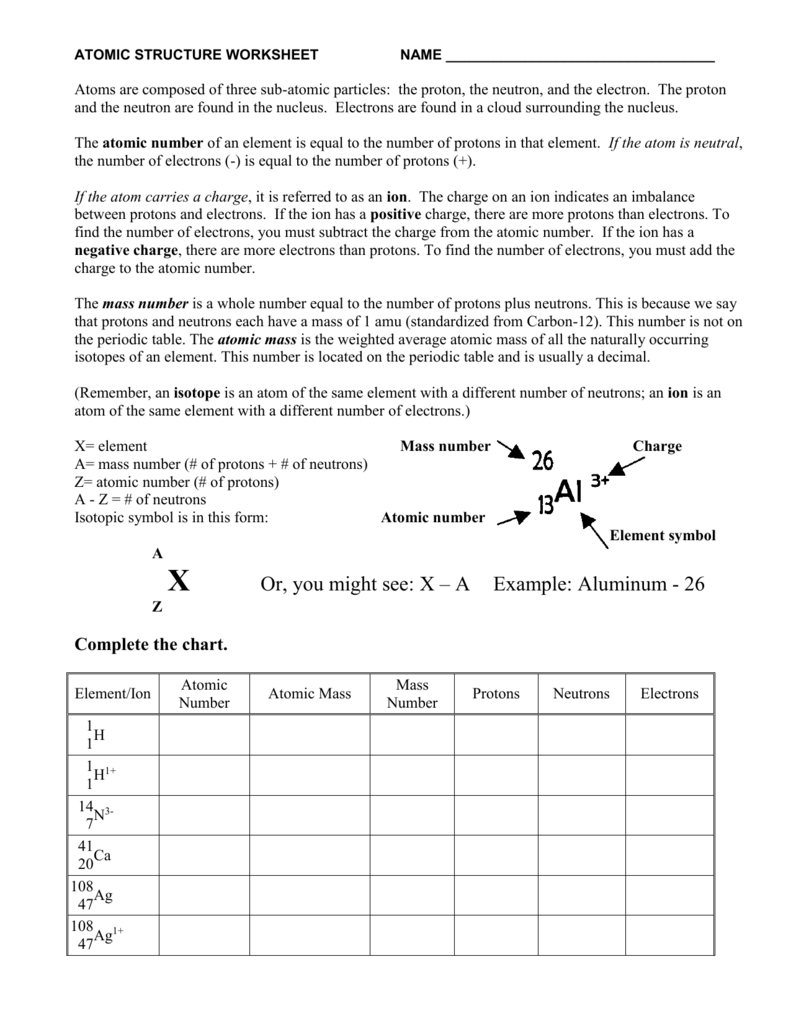
Atomic Structure Worksheet. Label the elements of an atom on the diagram below. What kind of charge does a proton have?

The electrons determine how atoms work together with each other. Atoms can share electrons to type molecules, that are particles made up of many atoms.

All atoms of an element have the same number of protons. But all atoms of that element can have completely different numbers of neutrons. This changes the mass variety of that atom, creating isotopes of that atom.

CBSE Class 9 Science Worksheet – Structure of Atom. Students can download these worksheets and practice them. This will help them to get higher marks in examinations.
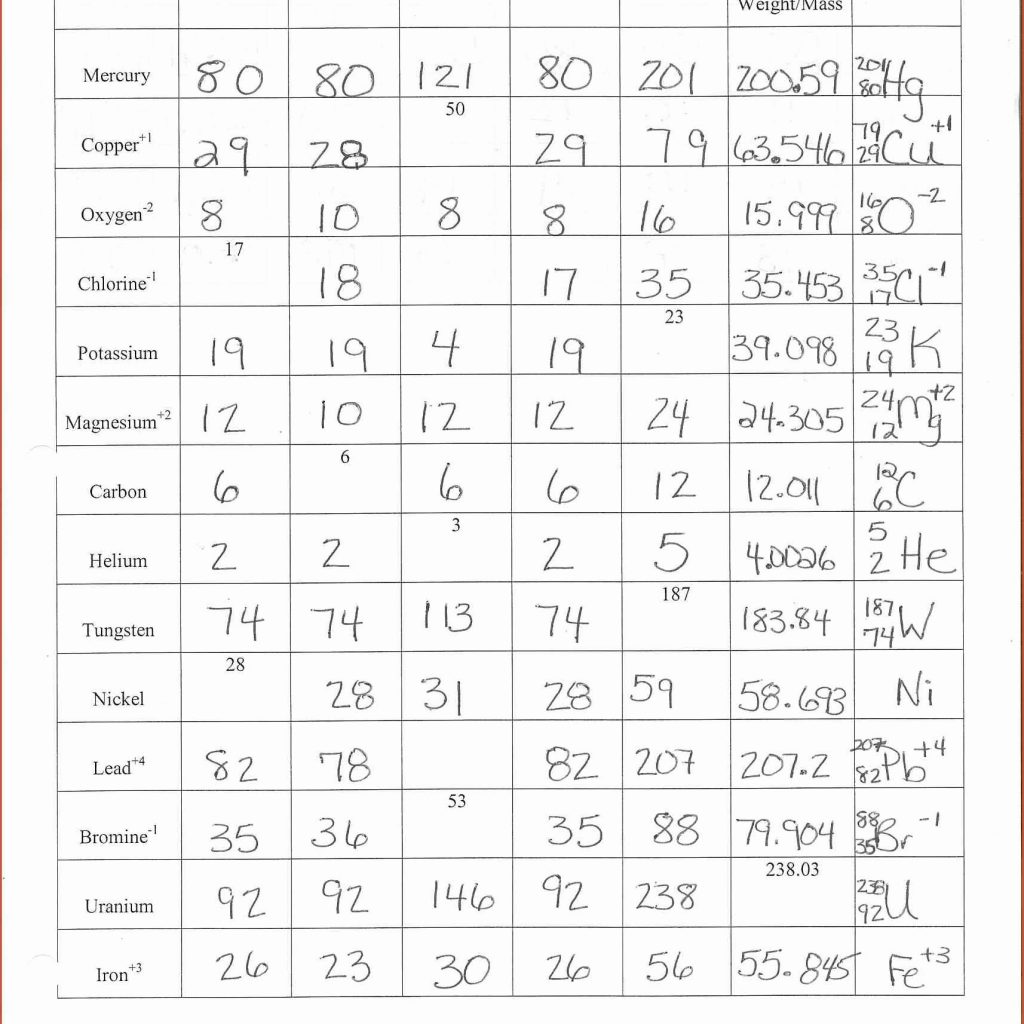
8th Grade Science Tutoringat eTutorWorld. Our skilled science tutors break down the topics via interactive one-to-one classes.