Nuclear Chemistry Worksheet K. Nuclear Chemistry Worksheet K Answer ship The Legal Hub. The α particle absorbs two electrons from the encircling matter to type a helium atom. Write the balanced nuclear equation for the response. Because this abundance just isn’t sufficient to help a series reaction, the uranium fuel should be no less than partially enriched in 235U, to a focus of about 3%, for it to have the ability to sustain a sequence response.
Smoke particles cut back the number of ionized particles and decrease the electrical present, which triggers an alarm. Another application is the “go-devil” used to detect leaks in long pipelines.
Nuclear radiation is throughout us in the environment. Low-level radiation is found in the oceans and waterways, the rocks and soils, the plant supplies and within the atmosphere surrounding the planet.
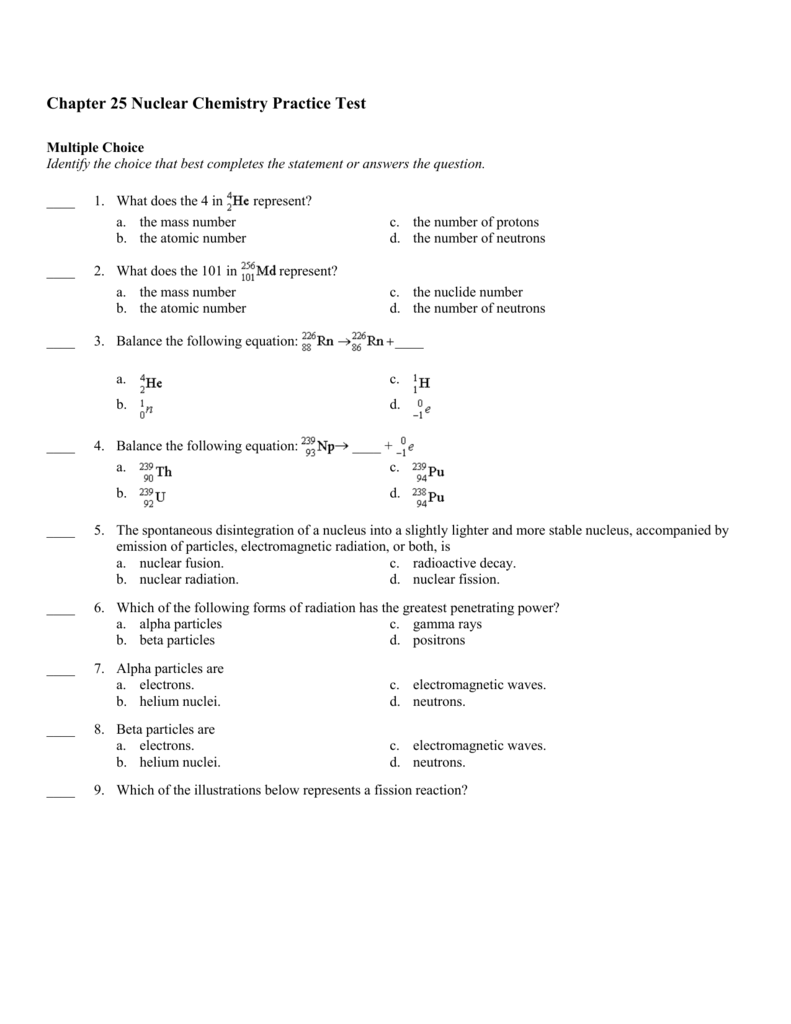
Nuclear Chemistry: Radioactivity & Forms Of Radiation
Using 10B and 252Cf, how would you synthesize a component with atomic number 103? A stable nuclide absorbs a neutron, emits an electron, after which splits into two α particles. Predict the most probably mode of decay and write a balanced nuclear response for each isotope.
Some properties of ionizing radiation are summarized in Table 20.three “Some Properties of Ionizing Radiation”. The depth of penetration of alpha, beta, and gamma radiation varies with the particle.
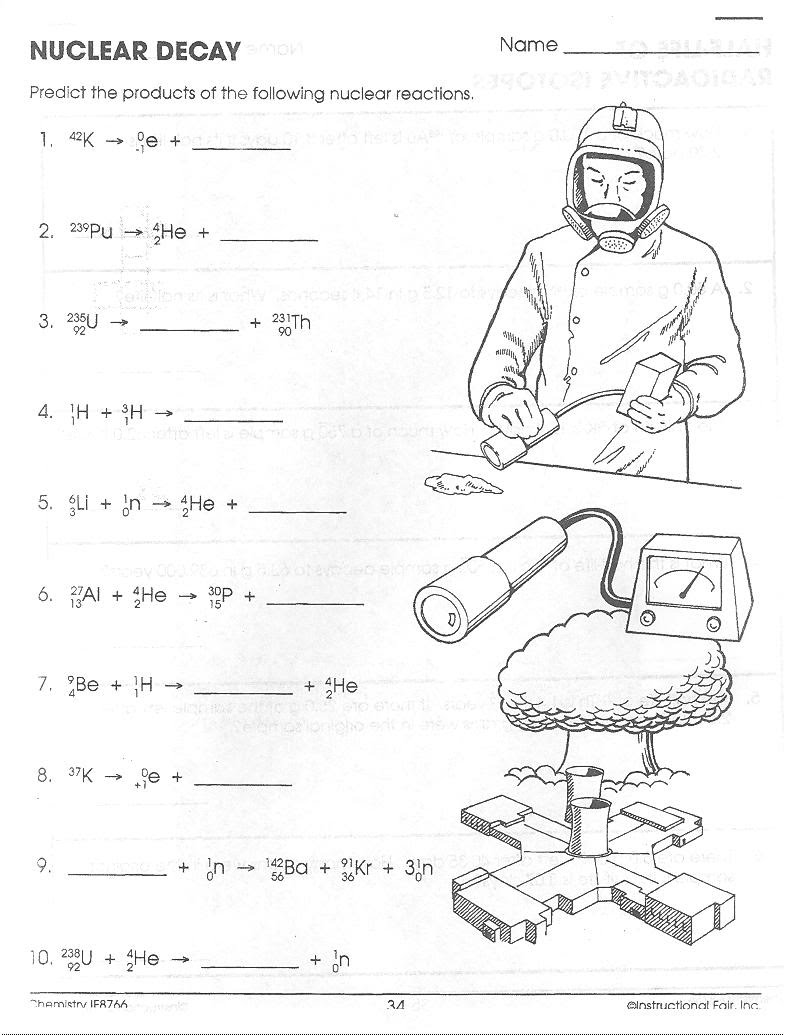
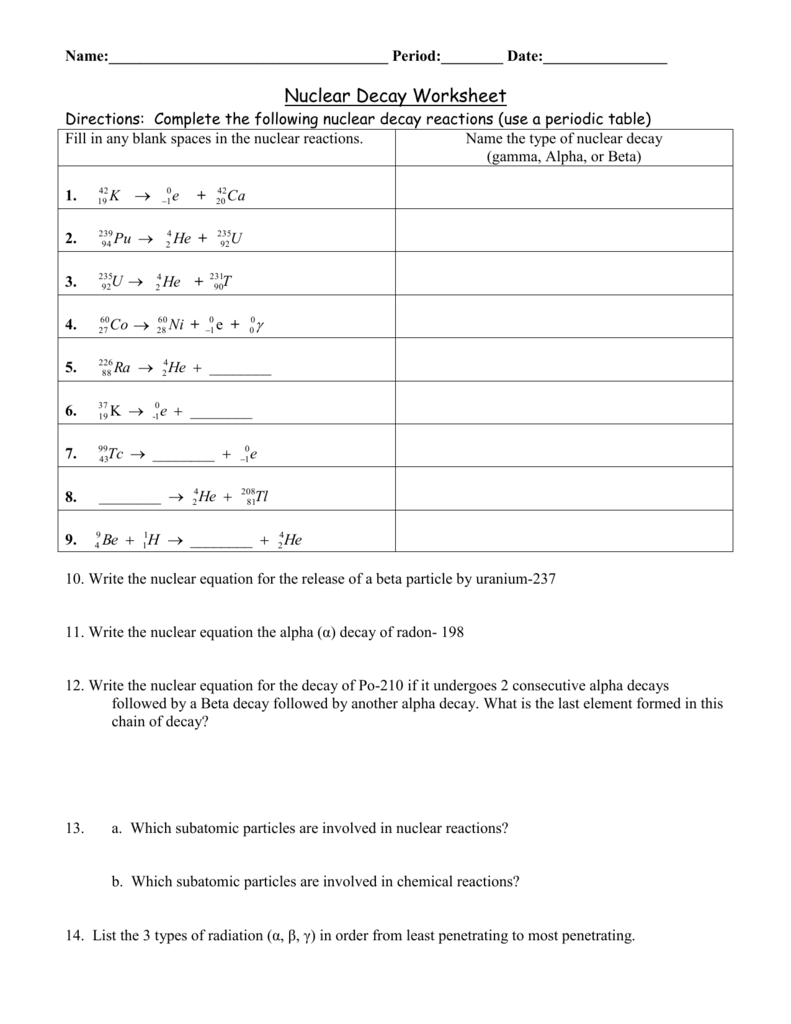
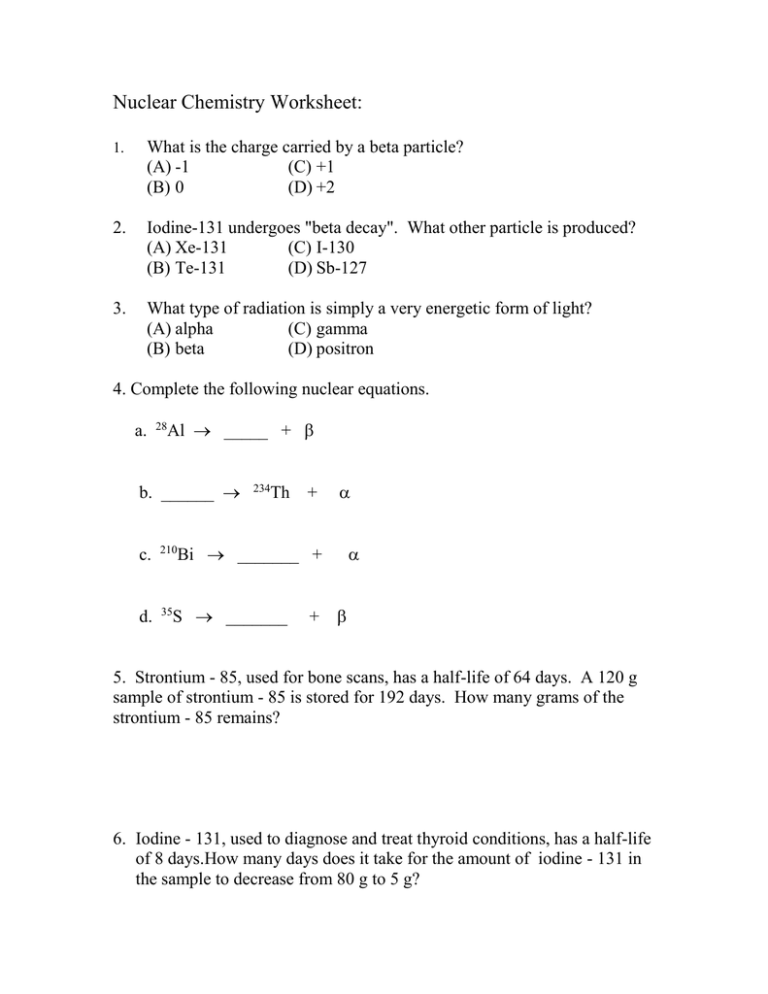
Nuclear Chemistry Unit Worksheet Packet
Please submit the worksheets on the Scholantis Portal web site. Please do NOT email me your work directly as it’ll get misplaced and I will not be able to mark it. Alpha particles can be stopped by very thin shielding however have a lot stronger ionizing potential than beta particles, X-rays, and γ-rays.
This radioisotope is a gamma ray producer with a half-life of 6 hours. When an organism dies it has a selected ratio by mass of carbon-14 to carbon-12 integrated in the cells of it is body. (The same ratio as within the atmosphere.) At the moment of demise, no new carbon-14 containing molecules are metabolized, therefore the ratio is at a maximum.
Answers
These reactions are liable for a lot of the monumental quantity of energy that’s launched as sunlight and photo voltaic heat. It takes several billion years, depending on the size of the star, to convert about 10% of the hydrogen to helium. A second major medical use of radioisotopes is medical imaging, during which a radioisotope is briefly localized in a specific tissue or organ, where its emissions provide a “map” of the tissue or the organ.
At much larger doses, nevertheless, their pure restore mechanisms are overwhelmed, resulting in irreversible injury. Because mice are biochemically far more just like people than are fruit flies, many scientists imagine that this mannequin also applies to humans. In contrast, the linear model assumes that all exposure to radiation is intrinsically damaging and suggests that stringent regulation of low-level radiation publicity is necessary.
Sjt Past Papers Pdf
Chances are that a large quantity of properties have had a quantity of of these gadgets put in as an early warning system in case of fire. What most shoppers do not know is that many of those units contain a small amount of americium-241 which emits alpha particles. By utilizing the radioactive properties of this material, smoke from a fire can be detected at a really early stage.

Until the Forties, uranium glazes were in style on ceramic dishware. One model, Fiestaware, had shiny orange glazes that would comprise as a lot as 20% uranium by mass.
Superheavy components, with atomic numbers near 126, may even be secure sufficient to exist in nature. In nuclear energy crops, nuclear reactions generate electricity.

To perceive the differences between nuclear fission and fusion. Calculate the annual radiation dose in rads a typical 70 kg chemistry scholar receives from the naturally occurring 40K in his or her body, which contains about one hundred forty g of potassium (as the K+ ion).
Zimbabwe Secondary College Books Pdf Free Download
The operate of this part is to guard staff from radiation produced by the nuclear reactions and to withstand the excessive pressures ensuing from high-temperature reactions. How many neutrons must an iron-56 nucleus absorb during a supernova explosion to supply an arsenic-75 nucleus? Write a balanced nuclear equation for the reaction.

The tissues most affected by massive, whole-body exposures are bone marrow, intestinal tissue, hair follicles, and reproductive organs, all of which contain rapidly dividing cells. The susceptibility of quickly dividing cells to radiation publicity explains why cancers are sometimes treated by radiation.
An analogous sequence of reactions converts nonfissile 232Th to 233U, which can also be used as a fuel for a nuclear reactor. Typically, about 8–10 yr are required for a breeder reactor to supply twice as much fissile materials as it consumes, which is sufficient to fuel a replacement for the unique reactor plus a brand new reactor. The products of the fission of 239Pu, however, have substantially longer half-lives than the fission products shaped in light-water reactors.

Medical imaging makes use of radioisotopes that trigger little or no tissue injury but are easily detected. One of an important radioisotopes for medical imaging is 99mTc. Some properties of different radioisotopes used for medical imaging are listed in Table 20.5 “Radioisotopes Used in Medical Imaging and Treatment”.

The decay of those daughter isotopes generates so much heat that the spent gasoline rods have to be saved in water for as lengthy as 5 yr earlier than they are often dealt with. Even then, the radiation levels are so high that the rods must be stored for many, many more years to permit the daughter isotopes to decay to nonhazardous levels. How to store these spent gas rods for tons of of years is a pressing issue that has not but been efficiently resolved.

The “Radioactive Decay of Candium” is one other half-life simulation but uses sweet that college students can then eat. Try this “Nuclear Chemistry” wordsearch with solutions .
The relative abundances of the elements within the recognized universe differ by greater than 12 orders of magnitude. For essentially the most part, these differences in abundance cannot be explained by differences in nuclear stability. Although the 56Fe nucleus is essentially the most stable nucleus known, essentially the most ample element within the identified universe just isn’t iron, however hydrogen , which accounts for about 90% of all atoms.

This equilibration course of types heavier components up to iron-56 and nickel-58, which have essentially the most secure nuclei identified. For a few years, the usual source for radiation remedy within the treatment of cancer was radioactive 60Co, which undergoes beta decay to 60Ni and emits two γ rays, every with an power of 1.2 MeV.
In addition, Earth’s oceans comprise an essentially inexhaustible supply of both deuterium and tritium, which suggests that nuclear fusion could present a limitless supply of vitality. Unfortunately, nevertheless, the technical necessities for a successful nuclear fusion reaction are so great that internet power generation by managed fusion has yet to be achieved. All nuclear reactors require a powerful cooling system to soak up the heat generated within the reactor core and create steam that is used to drive a turbine that generates electrical energy.
An unknown component emits γ rays plus particles that are readily blocked by paper. The sample also contains a considerable quantity of tin-104. Write a balanced nuclear equation for each response.

After yr week if you preliminary mass was one hundred fifty g 2 The decay especially for Sr-90 is 1237 min 1 If after half yr k. While small talk regarding Percent Composition Worksheet Answer Key. Writing Decay Equations Worksheet 1 Compare the decay modes of K-37 and K-42 according to Table N 2 Write the.

A Geiger counter is then used to detect the motion of the radioactive phosphorus-32 all through the plant. This data helps scientists understand the detailed mechanism of how vegetation utilized phosphorus to develop and reproduce.

Cosmic rays are additionally composed of excessive energy photons, and not all are prevented from reaching the earth’s floor. It is sensible that the higher you’re in altitude, the more you are exposed to cosmic radiation.
Do you count on Bi to have a massive number of secure isotopes? Nuclei with magic numbers of neutrons or protons are especially stable, as are those nuclei which are doubly magic.

Write a balanced nuclear response for the formation of every isotope. A view of the stays of Supernova 1987A, situated in the Large Magellanic Cloud, displaying the circular halo of expanding debris produced by the explosion.

Last, we discover the nuclear chemistry that takes place in stars, and we describe the function that stars play in producing many of the parts within the universe. Although most of the known parts have a minimal of one isotope whose atomic nucleus is stable indefinitely, all elements have isotopes which would possibly be unstable and disintegrate, or decay, at measurable rates by emitting radiation. Some parts have no steady isotopes and ultimately decay to other parts.

The answer—while but unknown—has extremely necessary penalties for regulating radiation exposure. Radiation of a high enough vitality to transfer some because it passes via matter to one or more atoms with which it collides. If enough power is transferred, electrons can be excited to very excessive power ranges, ensuing in the formation of positively charged ions.

The vitality released on this nuclear response is more than a hundred,000 instances higher than that of a typical chemical response, although the decay of 14C is a comparatively low-energy nuclear reaction. Because of their great penetrating capability, γ rays are by far essentially the most dangerous type of radiation when they come from a source outdoors the body. Alpha particles, nonetheless, are the most damaging if their source is contained in the physique because inside tissues take in all of their power.

At this degree of enrichment, a nuclear explosion is unimaginable; far larger ranges of enrichment (greater than or equal to 90%) are required for military applications similar to nuclear weapons or the nuclear reactors in submarines. Enrichment is achieved by converting uranium oxide to UF6, which is volatile and contains discrete UF6 molecules.

Another concept employs centered laser beams to heat and compress fuel pellets in controlled miniature fusion explosions (part in Figure 20.22 “Two Possible Designs for a Nuclear Fusion Reactor”). The fuel rods are made from a corrosion-resistant alloy that encases the partially enriched uranium fuel; managed fission of 235U within the fuel produces heat. Water surrounds the gas rods and moderates the kinetic vitality of the neutrons, slowing them to extend the chance that they’ll induce fission.

Radioactivity in nature comes from two main sources, terrestrial and cosmic. Terrestrial radioisotopes are discovered on the earth that got here into existence with the creation of the planet. Although some are long gone, some radioisotopes take a very long time to decay and turn into non-radioactive and are still round today.
In addition, we focus on a few of the purposes of nuclear radiation and radioisotopes, which have innumerable makes use of in medication, biology, chemistry, and industry. We begin this part by considering the completely different classes of radioactive nuclei, along with their attribute nuclear decay reactions and the radiation they emit. Quiz worksheet radioactive decay sorts impact research com.