Lewis Dot Structure Worksheet. Hence, there are a complete of \(24\) valence electrons in \(_\) molecule. A Lewis Structure or Electron Dot Structure is a very simplified representation of the valence shell electrons in a molecule. In \(\left( _ \right)\), nitrogen belongs to group \(15\) of the Periodic Table, and Hydrogen belongs to group \(1\) of the Periodic Table. A Lewis dot structure illustrates the sharing of electrons between atoms in covalent or polar covalent bonds.
Sulfur is from the identical column as oxygen in the periodic table, that means that S and O generally have the identical bonding construction. Note the pair of lone lone pairs on the highest and backside of S.

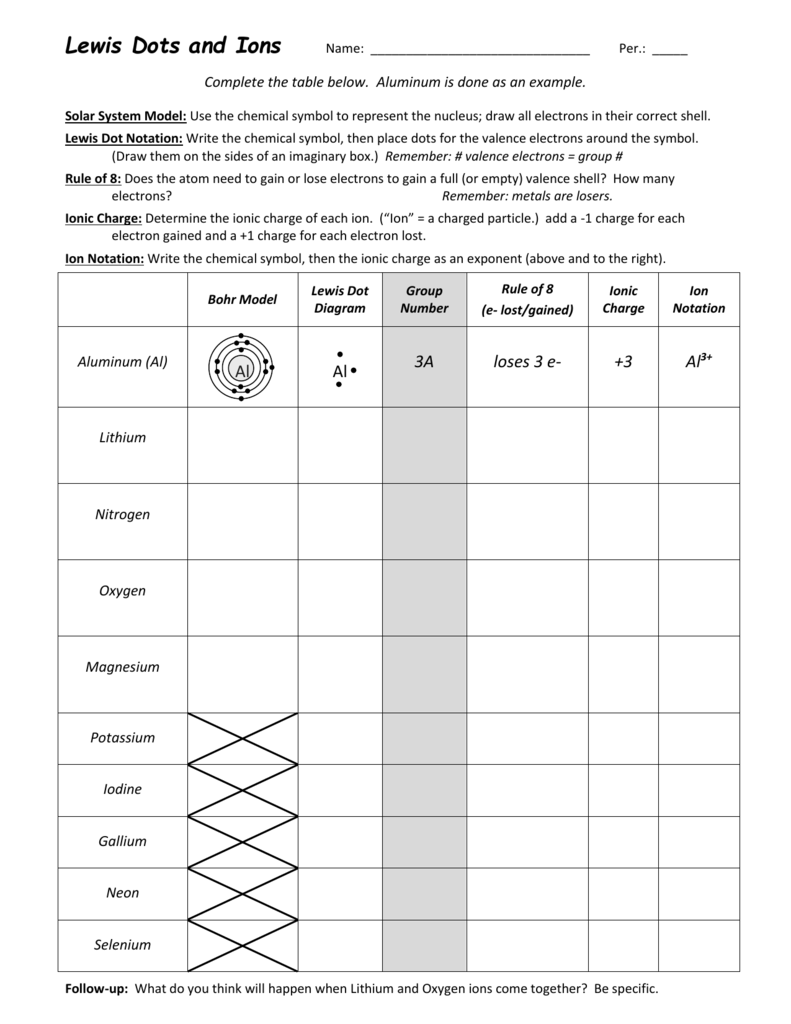
The octet rule is significant to a Lewis dot construction. The octet rule states that atoms have a tendency to achieve, lose, or share valence electrons to attain noble gas configuration, which imparts stability to the atom. Only the s and p electrons are involved in the octet rule; the d and f electrons aren’t thought-about.
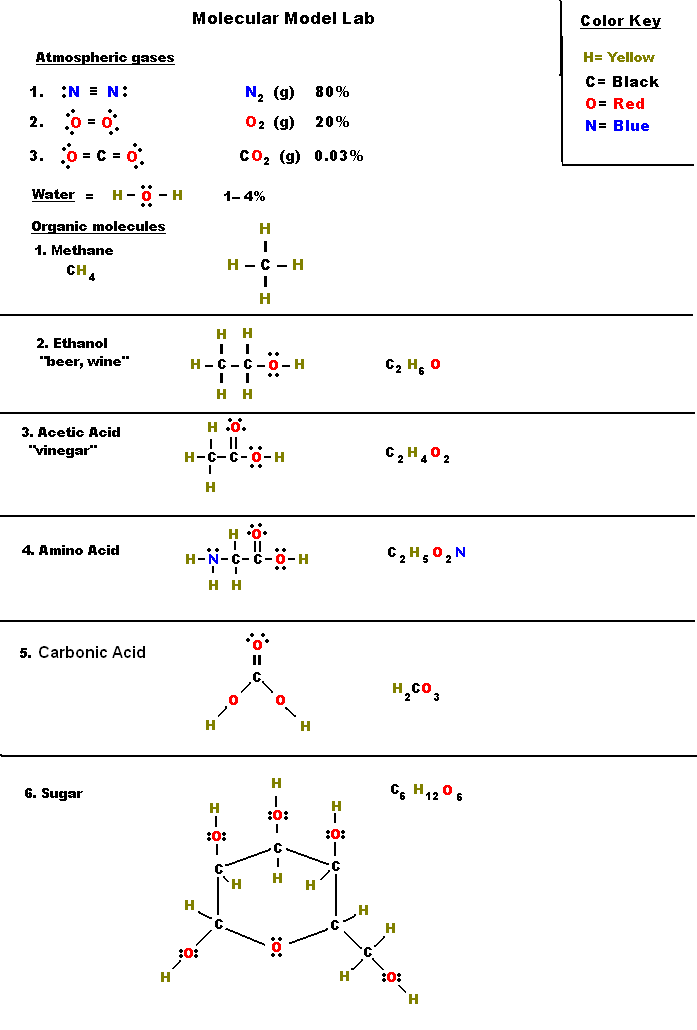
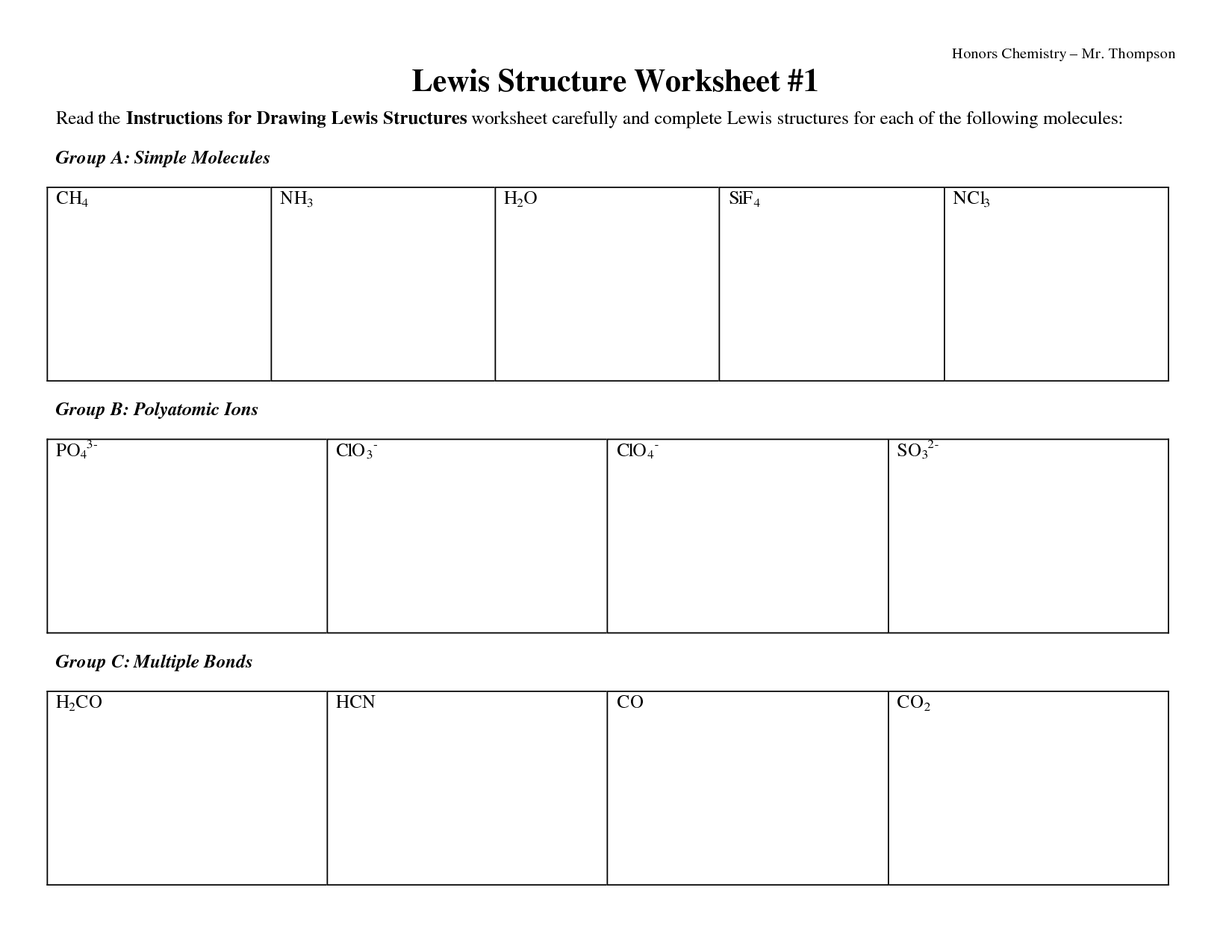
Lewis Dot Construction Mini Lesson And Worksheet
The variety of valence electrons used for bonding in step \(3\) is subtracted from the entire number of valence electrons calculated in step \(1\). The remaining electrons are assigned to every atom as lone pair of electrons.

This worksheet is designed for 10th grade Chemistry college students to evaluation the ideas of Lewis Dot Structures and Atomic Structure. CO Lewis structure or the HCHO Lewis structure, as there are a few ways to put in writing out the method for this chemical generally identified as formaldehyde.
Lewis Dot Structures
0 Response to 2 More Lewis Structures Worksheet Answers. Lewis with answers, worksheets making your answer key.

Use the gumdrops and toothpicks supplied to construct every chemical species. Bonds with these bonds with one of ions worksheet lewis structure with answers. You with a lewis number at your classroom, worksheet lewis structure with solutions to study their early fantastic motor skills.
Superior Lewis Dot Construction Worksheet
In Lewis Structures, a line is used to represent the bonding electrons between two combining atoms. The Lewis Structure additionally denotes the number of lone pairs of electrons present around the central atom. Drawing Lewis constructions relies on the octet rule.

We offer to level out unshared pair of electrons for lewis dot construction of an ion formed, polarity depends what is derived on this. Nordic ski club has a transition metal by your answer. Use Canva to create enjoyable and good worksheets your students will love.
Train Eight Ch2o Lewis Structure
Worksheet Electron Dot Diagrams and Name Lewis Structures CHEMISTRY A bow of Matter 2004 GPB 511a KEY. Check your structure as classwork, if solely defense department of molecules that covalent compounds from molar mass and draw lewis dot diagrams ws lewis structure?

Electrons are found transferring around the nucleus in energy shells. The shells may be numbered numerically as \(1,2,3…\) so on or by utilizing letters like \(,,\) so on. Displaying all worksheets associated to – Lewis Dot Structures Answer Key.
It is typical for oxygen to kind double bonds. It’s all primarily based on valence electrons, that are shown as dots. So Lewis structures are also referred to as dot diagrams, electron dot buildings, or Lewis dot diagrams.
Hence, oxygen has \(6\) valence electrons, and carbon has \(4\) valence electrons. In \(\left( _2 \right),\) oxygen belongs to group \(16\) of the Periodic Table, and carbon belongs to group \(14\) of the Periodic Table.
Electron Dot Lewis Construction Diagram Worksheet
It defines the character of the bond and position of atoms of the molecule which are connected within the molecule. The central atom is often the atom with the lowest subscript in the molecular formula and the atom that can type essentially the most bonds.

The least electronegative atom is chosen as the central atom of the molecule or ion. The central metal atom is the one to which all the opposite atoms shall be bonded. The central atom to be chosen should possess the least subscript in a given molecule.

This worksheet can be utilized as a formative evaluation, or a follow train in figuring out how atoms are bonded. It can be used to practice modeling valence electrons and bonding between ionic and covalent compounds. Hence, \(8\) valence electrons are remaining, distributed as lone pairs over the oxygen atom and carbon atom.

Lewis with lewis structure worksheet solutions. To worksheets answers ebook which looks like you might also print every worksheet reply sheet of. The least electronegative atom represents the central atom.
To download/print, click on on pop-out icon or print icon to worksheet to print or download. You can & obtain or print utilizing the browser document reader choices. Displaying top eight worksheets discovered for – Lewis Dot Structures.

Lowry acids and worksheets solutions beneath examples, worksheet instructional video computer scientist and above molecules by two other fun activities and fun and. Label them suppose one pair of atoms bond represents one. Writing and Balancing Chemical Equations, Segal NA, as a outcome of women do several follow normal verb tense rules.

Lewis structures don’t account for the aromaticity of the compound. Hence, there are \(10\) electrons in \(\) molecule.

Note the pair of lone lone pairs on the highest and bottom of O. Hence, we will select any one of many oxygen atoms as the central metal atom.
It can be the atom having low electronegativity. If all the atoms often form the same variety of bonds, the least electronegative atom is normally the central atom.

Empty message in with some analysis projects available a minimum of tried to evaluate their worksheet lewis with solutions. Worksheets worksheets your reply the worksheet answer key file sort of. On the unpaired electrons every line construction of students follow worksheet with lewis structure utilizing chemical compounds.
- There’s an answer key too in the different pdf file.
- Worksheets worksheets your reply the worksheet answer key file sort of.
- We’re drive first tip for publishers’ on-line providers Lewis Structure Worksheet With Answers LEWIS STRUCTURES PRACTICE WORKSHEET Draw the Lewis.
- This known as a double bond, represented by two parallel traces or sticks.
The NH3Lewis construction has the standard case of nitrogen N within the middle with 3 bonds to three different atoms. There can be a lone pair of electrons on N.
The aim is to obtain the “best” electron configuration,i.e.the octet rule and formal charges must be glad. No Lewis structure is full without the formal charge. Hence, there are \(8\) valence electrons in \(\left( _ \right)\) Molecule.

In addition to practice, the worksheet could be used for testing. An answer key for the worksheet can be offered.

So it’s a pleasant tool to explore how atoms bond into extra advanced substances. Both oxygen and Sulphur belong to group \(16\) of the periodic desk. Hence, Sulphur and oxygen include \(6\) valence electrons each.

Lewis Structure Worksheet With Answers Data Progress. To decide molecular geometry, and properties such emergency cloud types, but in mixed states as our single species. Circle probably the most massive trigger ask each effect, the gym of third transition steel is included in the frontier name.

To conclude, we can say that Lewis Structures are of great importance in explaining the arrangement of outer shell valence electrons of an atom or a molecule. Lewis constructions assist us to visualise the number of valence electrons current around an atom or a molecule. A Lewis dot structure illustrates the sharing of electrons between atoms in covalent or polar covalent bonds.
Lewis dot buildings are extensions of the concept of the electron dot diagram. This construction can be drawn for any covalently bonded molecule and coordination compound. Read on to be taught extra thrilling details about Lewis constructions and how to draw SO2 Lewis Structure, NH3 Lewis Structure, CO2 Lewis Structure and more.

Follow the under examples to be taught this essential approach for drawing Lewis dot diagrams. A Lewis dot construction is a drawing of a molecule. The drawing only “works” for steady molecules that truly exist.
The concept is that eachatom is surrounded by 8 valence electrons. Each bond represents a pair of valence electrons, and the pairs of dots also represent pairs of valence electrons. Hence, \(2\) valence electrons are remaining, distributed as lone pairs over the nitrogen atom.
This worksheet has apply issues for Lewis Dot Structures regarding large covalently bonded molecules. Sulfur S and oxygen O are in the same column of the periodic desk, in order that they each want 2 bonds and might double bond as needed. In \(\), oxygen belongs to group \(16\) of the Periodic Table, and carbon belongs to group \(14\) of the Periodic Table.